Background
We develop advanced cell culture systems using micro- and nano-fabrication techniques to provide a better "in vivo-like" cell culture environment to brain cells in a dish. Currently, we have projects available in the following research areas.
Research Interests:
Neuro-Bioengineering; Neurodegenerative diseases and disorders; 3D neuronal tissue culture tools; Nanomaterials; Nanoparticles, Bio-Hydrogels; Microfluidics; Magnetic microdevices; BioMEMS; Mathematical Modeling; Biophysical Models
Links:
Google Scholar, Research Gate
Research Interests:
Neuro-Bioengineering; Neurodegenerative diseases and disorders; 3D neuronal tissue culture tools; Nanomaterials; Nanoparticles, Bio-Hydrogels; Microfluidics; Magnetic microdevices; BioMEMS; Mathematical Modeling; Biophysical Models
Links:
Google Scholar, Research Gate
Extracellular Vesicle Diagnostics for Brain Diseases (Exodiagnostics)
|
Extracellular vesicles are lipid cargos, approximately a thousand times smaller than the size of a human hair. These small cargos get released from the cell body with the purpose of removing toxic signals. However, they also carry RNAs or peptides that can be used in diagnostics to detect early pathological states of a single cell or cell population. The small size of extracellular vesicles and the large variety of different cells from where the vesicles originate makes precise disease diagnosis challenging. Hence, the Kunze Lab is developing microsystem-based advanced tools to refine using extracellular vesicles for the detection of neurological disease states.
|
Nanomagnetic Force-based cell engineering
|
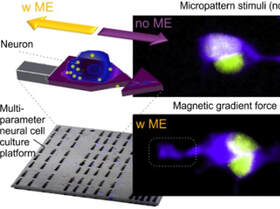
The brain is the softest organ in humans. During the folding of the human cerebral cortex, neurons situated in the same cell tissue layer but in different global locations present different cellular morphologies. Although known, this phenomenon is hardly addressed in today's cell culture assays. The Kunze Lab has pioneered two approaches, where either (i) magnetic nanoparticles get used to mechanically modulate neurons from the inside of the cell body, or (ii) microfeatures shape and bend cell growth from the outside. Both approaches are scale-able and use on-chip technology, allowing us to ask how bending and modulating forces alter calcium signaling or may impact the propagation of typical Alzheimer's disease hallmarks.
|
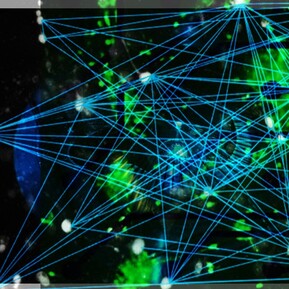
Electrophysiology and Advanced Brain Network Analytics
|
To better understand the underlying functionality of brain networks, we aim to integrate the recording of electrophysiological signals from our engineered brain cell cultures. Through this process, we often capture over twenty thousand data points per second, generating vast amounts of data sets. To analyze these big data sets efficiently, we collaborate with faculty members in the Department of Mathematical Sciences and apply new ways to extract underlying signal patterns, which can tell us more about the connectivity and physiological function of the neuronal networks.
|
Life-cell, Low-cost, Integrated Imaging Systems
|
Growing primary neuronal cells requires a dedicated cell environment as close as possible to the physiological state of a brain. Although a variety of systems do exist to keep neurons alive for several months in Petri dishes or micro devices, access to tools for modulation and imaging is limited and can be costly. Hence, the Kunze Lab is designing and building low-cost integrated imaging solutions, which allow us to capture cellular signals at high-speed in remote and low resource environments. This approach brings further advantages to training students in data and network analysis and integrating these experiences into the classroom, as well as fostering collaborations with remote off-campus research labs.
|
Neurodegenerative diseases on-chip
|
A significant hallmark of Alzheimer's disease is the accumulation of hyper-phosphorylated Tau proteins. In vitro, adding a phosphatase inhibitor can induce this form of disease state; however, within the same cell culture, all cells are exposed to the same inhibitor concentration. The Kunze Lab designs and employs microfluidic environments with the ability of complex gradient formations. With these gradients, we can induce co-pathological states of hyper-phosphorylated Tau within the same neural cell population. We also formed an on-chip astrocyte-based amyotrophic lateral sclerosis (ALS) model in the microfluidic-based cell culture platform. The co-pathology here consisted of genetically modified astrocytes (SOD: superoxide dismutase depletion), which were in metabolic contact with healthy cortical neurons.
|
Microfluidic-based cell culture platforms (Neurofluidics)
|
Cell cultures in the dish are critical assays in bioscience, yet they do not provide enough complexity to resemble the cellular environment. Most cells are highly susceptible to their environment. Thus, a slight change in the cellular environment can easily modify cellular behavior, leading us in the wrong direction when it comes to developing cell-based therapeutics. Using microtechnology, specifically microchannels cast into biocompatible polymers (microfluidics), the Kunze Lab can build complex cellular environments where cells are exposed to gradients, three-dimensional tissue-like architecture, or local differences in cell density.
|
(c) 2015 - 2024 Kunze Lab